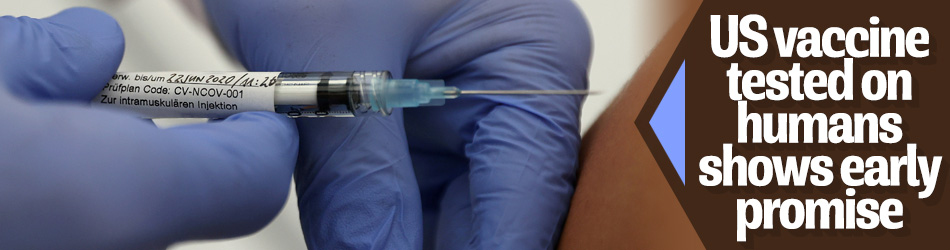
A Massachusetts-based biotech company has been testing a possible vaccine against the novel coronavirus which is showing early signs of success.
Moderna Therapeutics announced an estimated start date for phase 3 trials, its final phase of testing.
VOLUNTEERS WILL BE MONITORED FOR A YEAR
The trial involved 45 volunteers aged 18 to 55 who received one of three dosage levels of the vaccine, which was given in two injections about a month apart.
Researchers measured virus-recognizing antibodies in all participants and detected levels similar to or higher than those found in the blood of people who have recovered from coronavirus.

It is not clear how long the immune response will protect against coronavirus, but volunteers will be monitored for a year to find out. Some participants experienced mild side effects such as fatigue, chills, headache and weakness.
Moderna’s vaccine, which is being co-developed with the US National Institute of Allergy and Infectious Diseases (NIAID), began safety testing in humans in March. Phase 3 tests will begin on July 27 and will involve 30,000 people, according to a May 18 press release.

Half of the participants will be a control group who will receive placebos. This large clinical trial is expected to be completed by late October.













